Recent headlines introduced Manhattan aesthetician, Joey Grant Luther, accused of injecting his clients with counterfeit Botox. He was arrested, but only after several patients experienced significant complications, some requiring hospitalization.
Clients experienced adverse reactions such as: double vision, difficulty chewing, difficulty swallowing, palpitations, and slurred speech. Luther complained to his supplier that clients were experiencing complications from “contaminated” product. However, the complications experienced by Luther’s clients are typical for excess or off-target dosing of the active ingredient.
Layers of illegality
There are several layers of alleged illegality in this story:
- The imported drugs were not approved for use or sale in the US.
- Botox is an FDA regulated drug and can only be given by a provider with appropriate licensing.
- Mr. Luther had no license to give prescription medication.
- He knew the product was counterfeit but explicitly promoted it as the real thing.
- He continued to use the illegal product even after he was aware of the adverse effects on his clients.
Mr. Luther is facing serious charges, and potentially serious jail time.
According to the US Attorney’s Office of the Southern District of New York:
LUTHER, 54, of New York, New York, is charged with one count of wire fraud, which carries a maximum sentence of 20 years in prison; one count of dispensing of a misbranded drug while held for sale, which carries a maximum sentence of one year in prison; one count of holding counterfeit drugs for sale and for dispensing, which carries a maximum sentence of 10 years in prison; one count of receiving misbranded drugs in interstate commerce and delivery or proffered delivery thereof, which carries a maximum sentence of three years in prison; and one count of smuggling, which carries a maximum sentence of 20 years in prison.
Botulinum toxin is sold in various formulations under various brand names in US and abroad. The most common is “Botox,” a registered trademark of Allergan. The product used by the Mr. Luther was allegedly a counterfeit drug mislabeled as Botox. The various brands/formulations are approved for a variety of therapeutic and cosmetic indications. For most of this article I will ignore the differences between the various formulations and tradenames and use the generic term “botulinum toxin.”
Clostridium botulinum, the villain and hero of the story
Clostridium botulinum is an anaerobic bacterium notorious for producing a potent neurotoxin. Eating food contaminated with Clostridium botulinum can cause widespread muscle weakness/paralysis, a condition known as botulism. Botulism can cause death due to paralysis of the respiratory muscles.
Muscle contractions are activated by nerves. Nerves activate muscles via the neurotransmitter acetylcholine. The culprit in botulism is a class of proteins, hereafter referred to as botulinum toxin. These toxins prevent release of acetylcholine from these nerves. In the absence of acetylcholine, the muscle is unable to contract, thus botulinum toxin causes muscle weakness or paralysis. Botulinum toxin is incredibly potent, possible the most potent toxin known. The effects of botulinum toxin are prolonged but temporary, lasting weeks to months.
Under the proper conditions, Clostridium botulinum bacteria produce spores. The spores themselves are not toxic, but are very difficult to kill. If food is not sterilized properly and stored in low-oxygen conditions, the spores can germinate into bacteria and produce botulinum toxin. If the infected food is consumed, the toxins cause possible fatal botulism. Prevention of botulism is the reason preserved foods must be properly prepared, and why you should never consume food from “bulging” cans.
Medical use of botulinum toxin
A truly innovative physician named Alan Scott deserves most of the credit for the medical use of botulinum toxin. Dr. Scott, an ophthalmologist, was interested in treating a condition called strabismus, or misalignment of the eyes. Each eye is moved back-and-forth and up-and-down by 6 muscles. For a variety of reasons, the eyes can become misaligned. Dr. Scott understood that botulinum toxin weakened muscles and reasoned that if he could strategically weaken some of the eye-movement muscles he might be able to bring the eyes into alignment.
What Dr Scott and a small group of collaborators achieved with limited time and resources was remarkable. The toxin was provided by an outside scientist. Dr. Scott developed processes to manufacture, and assay a stable formulation of the toxin. He performed appropriate animal studies. He set up a small scale production facility utilizing Good Manufacturing Practice with colleague Dennis Honeychurch.
The extraocular muscles targeted for treatment with botulinum toxin are small, and difficult to see without surgical exposure. In parallel with the development of the drug, Dr. Scott developed a technique for localizing the muscle using electromyography and injecting the drug with the same needle, obviating the need for surgical exposure. The first human was treated for strabismus in 1977, with good results. The first case series was published in 1980. Dr. Scott produced the drug under the name “Oculinum.” For years he manufactured the drug and provided it to colleagues for a nominal fee. Clinicians found other uses for the drug.
Expansion of botulinum toxin usage
In addition to strabismus, Dr Scott recognized the potential use for a treatment called essential blepharospasm. Patients with this condition develop uncontrollable, forceful closure of the eyelids and facial muscles around the eyes, which can be a physically and socially disabling condition. As Dr Scott predicted, the condition responded very well to treatment with botulinum toxin. This paved the way for the use of the drug for a variety of conditions related to dysfunctional muscle contraction.
Due to the rigors and liability of manufacturing and distributing the drug Scott sought, and initially had difficulty in finding, a partner pharmaceutical company. Ultimately, a then small drug company, Allergan, saw the potential of the drug and bought the company from Dr Scott. Dr. Scott died in 2021.
Global market
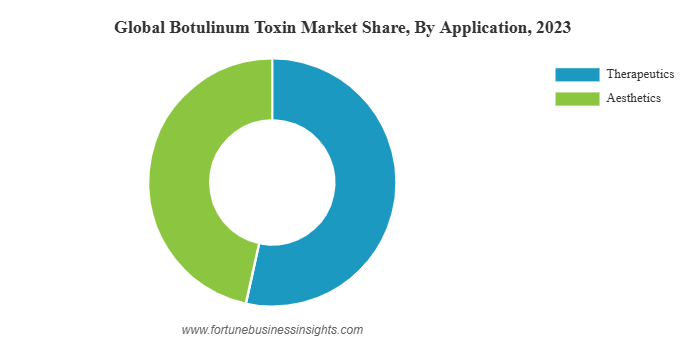
The global market for botulinum products was valued at $8.14 billion in 2023 and growing. The products are produced by multiple companies under a variety of names. Products using botulinum toxin are FDA approved for a variety of indications including strabismus, blepharospasm, muscle spasticity, chronic migraine, and axillary hyperhidrosis (excess armpit sweating). The global market for botulinum products also has a large market for aesthetics. Just over half of the market is for therapeutic use and the remainder for aesthetic indications.
Misuse of botulinum toxin
Botulinum toxin is no joke. The lethal dose is miniscule.
When used responsibly botulinum toxin is very safe. Because of extreme potency and potential for off-target muscle paralysis, careful control of dosage and injection technique is critical. Unfortunately, due to recklessness or cluelessness, some have used these products frivolously.
In the hands of Manhattan aesthetician Joey Grant Luther, the excess complications could have been due to mislabeled concentration of the counterfeit Botox, poor technique by the untrained, unlicensed provider, or both.
There have been other similar abuses. In 2004, a physician in Florida overdosed himself and 3 others This particular physician had lost his license due to previous infractions. He purchased botulinum toxin sold for laboratory-use-only and, despite warnings from his assistant, he treated himself and 3 others for cosmetic uses with enormous over-doses. Concentrations measured in their blood were 21 to 43 times the estimated lethal dose. Further details were revealed in a 2006 report. Fortunately, they all survived due to medical care including administration of antitoxin, and protracted hospitalization with “prolonged mechanical ventilation.” In other words, these patients were paralyzed to the extent they could no longer breathe and would have died if not supported with a ventilator.
Buyer beware
In 2024 Centers for Disease Control reported 19 people in 9 states with “harmful reactions” after treatment with counterfeit drug or by “unlicensed or untrained individuals and/or in a non-healthcare setting…” Nine were hospitalized.
A later CDC report provided further guidance. Patients were encouraged to investigate the credentials of providers, and advised to inquire whether or not the product is the FDA approved version obtained from a reliable source. Patients were urged to seek urgent medical attention if they experience symptoms of unintended muscle weakness, including: blurry or double vision, drooping eyelids, difficulty swallowing, difficulty breathing, or muscle weakness. I would add a caution that patients should be especially vigilant about cosmetic treatments done outside a medical setting, such as a spa or at-home treatment.

